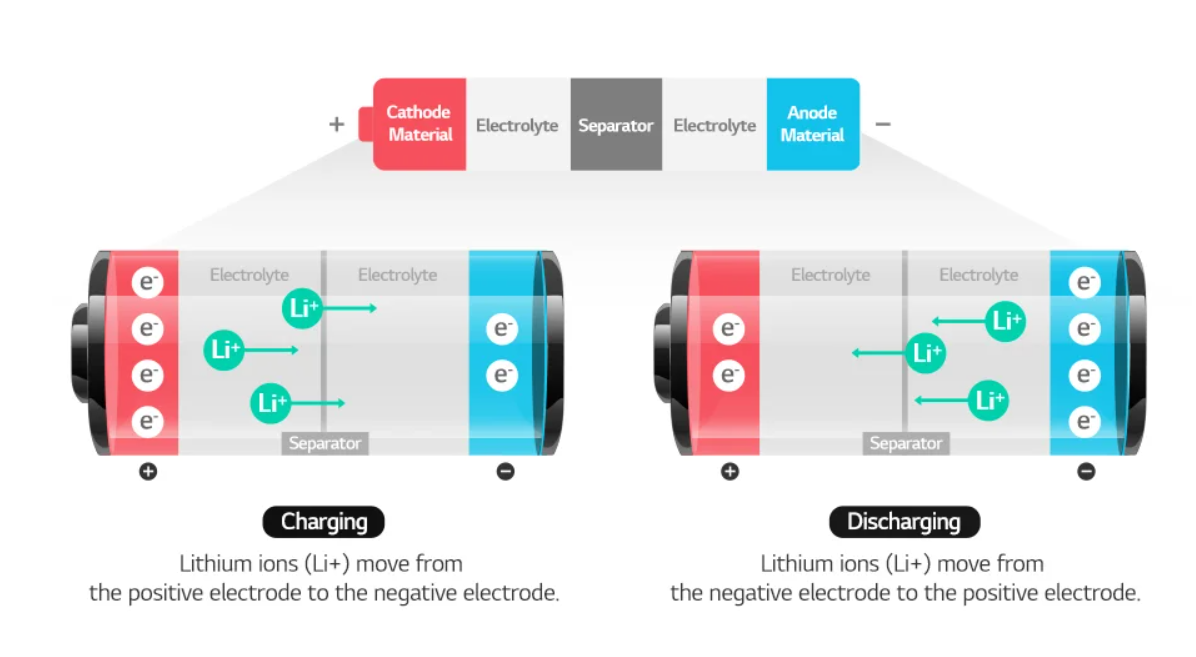
Lithium ions in lithium-ion batteries move between cathode and anode, causing a chemical reaction and generating electricity.
A battery is charged when lithium ions move from cathode to anode, and is discharged when the lithium ions move back to the cathode as it releases energy. For this process to happen, the battery needs an electrolyte through which lithium ions can pass, and a separator to keep the cathode and anode separated. In general, the cathode, anode, separator, and electrolyte make up the four major components of a lithium-ion battery.
Cathode
In a lithium-ion battery, lithium ions enter into the cathode, which can be thought of like a house for lithium ions. Lithium is a perfect cathode material since it tends to lose electrons and turn into a positive ion. However, since elemental lithium is unstable, lithium oxide, a combination of lithium and oxygen, is used instead.
The cathode determines the capacity and voltage of a battery, which are the critical components of battery performance. Battery capacity improves as the lithium proportion increases. The voltage is determined by the potential difference between the electrodes. Therefore, the potential value due to the structure of the cathode and anode influences voltage. Recently, as the demand for high-performance cathode materials increases, various cathode materials like NCA (nickel/cobalt/aluminum) and NCMA (nickel/cobalt/manganese/aluminum) are under development.
Anode
Anode materials store and release lithium ions from the cathode, allowing current to flow through an external circuit. When the battery is charged, lithium ions are in the anode. When the anode and cathode are connected with a conducting wire, lithium ions move from the anode to the cathode via the electrolyte, while the electron separated from the lithium ions move along the wire, generating electricity. You can think of it as lithium ions leaving home and generating electricity while working.
Graphite can store many ions and is mainly used for anode materials. However, as the process of storing and releasing lithium ions is repeated, the structure of graphite changes, and the number of ions that can be stored decreases, reducing battery life. That is why the next-generation anode materials, such as silicon with a large capacity and can accelerate charging, are currently being developed.
Electrolyte
Electrolyte is a medium that helps lithium ions move between the cathode and anode inside the battery. It can be thought of as a form of transportation that lithium ions take to commute to work. The electrolyte must have high ionic conductivity for the smooth movement of lithium ions, as well as high electrochemical stability and high flash point for safety. Also, it is necessary to prevent the electrons from entering the electrolyte and make them only move along the external conducting wire.
Currently, liquid electrolytes are widely used for this purpose. However, research is now being carried out on solid or gel-type electrolytes with better safety and performance.
Separator
As it is essential to completely separate work from home to achieve a good work-life balance, a separator prevents the cathode and anode from having physical contact. Tiny pores on the separator allow lithium ions to move. In other words, the separator blocks the contact between the two electrodes but allows ions to move through.
The separator needs to have high electrical insulation and thermal stability for safety, and it must also automatically block the movement of ions at a temperature above a certain level. Currently, polyethylene (PE) and polypropylene (PP) are widely used as separators. A study is currently being conducted on making the separator thinner to miniaturize the battery.
So far, we have looked at the four main components of a lithium-ion battery and how they work. Lithium-ion batteries have made our lives as convenient as it is today, and yet, even at this moment, more studies are being carried out to overcome their limitations. LG Energy Solution is leading the industry and is heading the development of next-generation batteries. On <Battery Day 2021> held in April 2021, LG Energy Solution announced plans to commercialize lithium-sulfur batteries in 2025 and all-solid-state batteries between 2025 and 2027.
In September 2021, LG Energy Solution, through a joint study with the University of California San Diego (UCSD), announced that it had overcome the technical limitations of the all-solid-state battery, which could only be charged above 60℃. The company has developed a long-life all-solid-state battery technology that can be charged at room temperature (25℃) at a fast rate. The technological breakthrough was recognized and published in the renowned scientific journal, Science. Even now, many people are striving to commercialize next-generation batteries, and we are, in fact, a step closer to the future without even noticing it.
